Innovating Patient Care
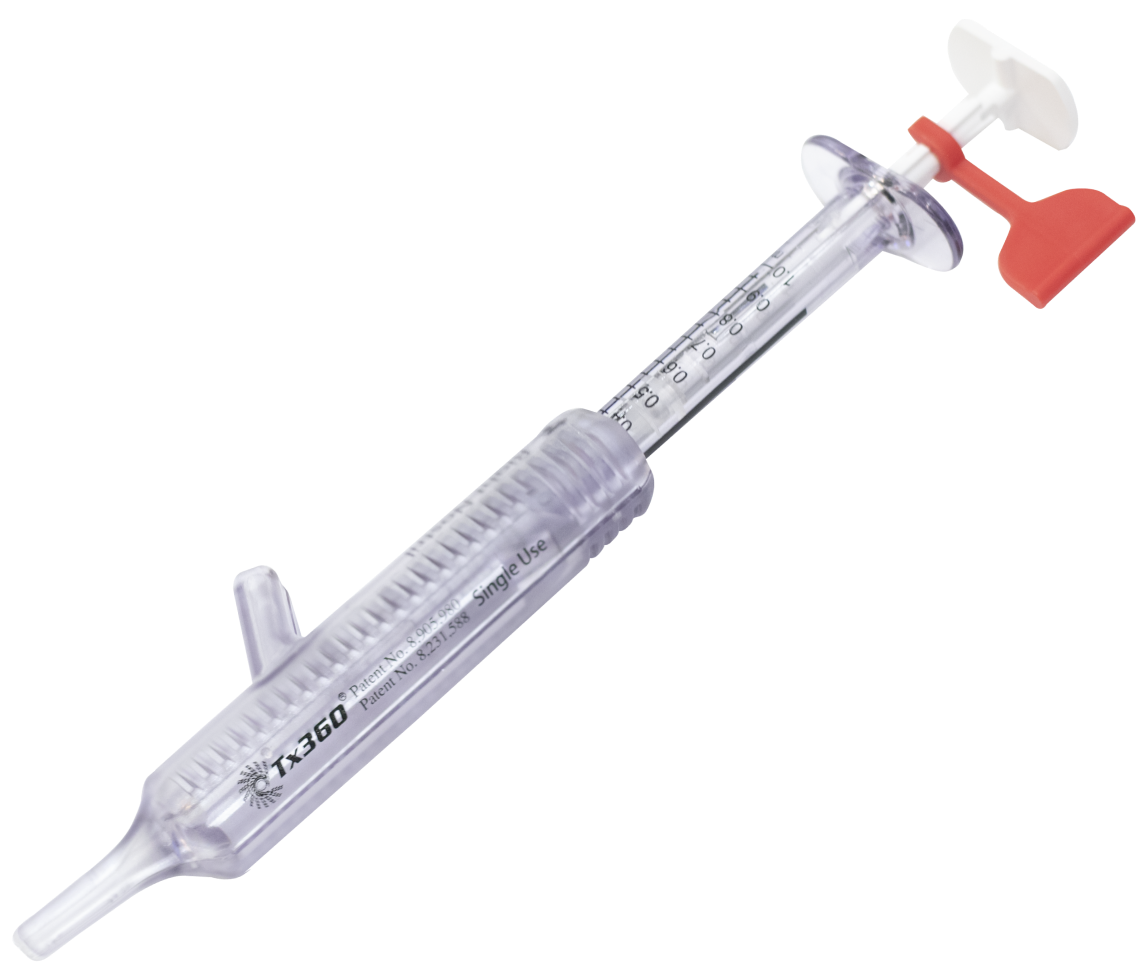
Tx360® Nasal Applicator
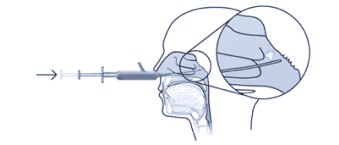
At the forefront of innovation, through its unique design and superior functionality, Tx360® is making groundbreaking strides in targeting the SPG
U.S. Patent No. 8,231,588/8,905,980
Precise targeting, for optimal results
The only FDA cleared device of its kind
Patented technology targets the SPG nerves to alter its signals
Delivering medication precisely and consistently to the SPG, with ease, every time
Minimal medication,
Maximum effect
Watch How the Tx360® Works
Understanding Tx360®: Revolutionizing SPG Treatments
About Tian Medical
We merge pioneering science with medical excellence.
Founded by Dr. Tian Xia, a thought leader in Interventional Pain Management, Tian Medical’s flagship invention, the Tx360® Nasal Applicator, is a testament to the commitment of offering a modernized and innovative technology to age-old and new procedures.

Your Health, Our Mission:
Trusted Physicians, Transformative Care
I am a medical professional
Professional Partnerships Welcome
I am a medical professional
Professional Partnerships Welcome
I am an individual
Learn more about the Tx360®
Thank you for your interest in Tx360
To nominate your provider to be trained on Tx360
Hear from Our Community:
Real Success Stories with
Tx360®
We found the procedure to be very easy to learn and quick to administer while providing good outcomes.
Headache Specialistc
Given the overwhelming success of this treatment, we plan to continue using the Tx360 in our clinics and in the Urgent Care.
Neurologistc
The procedure has allowed me to stay away from using over-the-counter medications and prescriptions, longer than anything else I’ve tried.
Patientc
SPG procedures have given me the biggest window of relief, which has reduced the amount of time I’ve had to take off work.
Patientc
The application of the nasal catheter isn’t painful at all. I would have these treatments again.
Patientc
We found the procedure to be very easy to learn and quick to administer while providing good outcomes.
Headache Specialist
Given the overwhelming success of this treatment, we plan to continue using the Tx360 in our clinics and in the Urgent Care.
Neurologist
The procedure has allowed me to stay away from using over-the-counter medications and prescriptions, longer than anything else I’ve tried.
Patient
SPG procedures have given me the biggest window of relief, which has reduced the amount of time I’ve had to take off work.
Patient
The application of the nasal catheter isn’t painful at all. I would have these treatments again.
Patient